Abstract
Background: HLA-identical sibling hematopoietic stem cell transplant (HSCT) using myeloablative chemotherapy conditioning is a proven cure for sickle cell disease (SCD), but is associated with serious short and long-term toxicities. Additionally graft versus host disease (GVHD) can complicate care post-HSCT and contribute to mortality. Given these concerns many pediatric hematologists and families are reluctant to pursue HSCT for SCD. Nonmyeloablative HSCT resulting in stable mixed chimerism has been demonstrated to abrogate the SCD phenotype in adults. Data on outcomes of this approach to decrease toxicities and achieve cure in children remains scarce.
Objective: To evaluate event-free survival (EFS), toxicity, health-related quality of life (HRQL), and transfusion burden among pediatric patients with SCD using a chemotherapy-free nonmyeloablative regimen.
Methods/design: Children and young adults with SCD were prospectively enrolled in the Sickle transplant Using a Nonmyeloablative approach (SUN) multicenter clinical trial (NCT03587272). Six Sickle Cell Transplant Advocacy and Research Alliance (STAR) sites in the United States and Canada enrolled patients. The conditioning regimen consisted of alemtuzumab IV (0.03mg/kg on Day -7, 0.1mg/kg on Day -6, 0.3mg/kg on Days -5 to -3), low-dose total body irradiation with gonadal shielding (300 cGY on day -2), peripheral blood stem cells from an HLA-identical sibling, and sirolimus for at least one year. Baseline HRQL was compared with scores at day +30 and day +100 post-HSCT using the PedsQL and PROMIS measures. EFS was defined by any of the following events: death, graft failure (myeloid donor chimerism <10%), or GVHD.
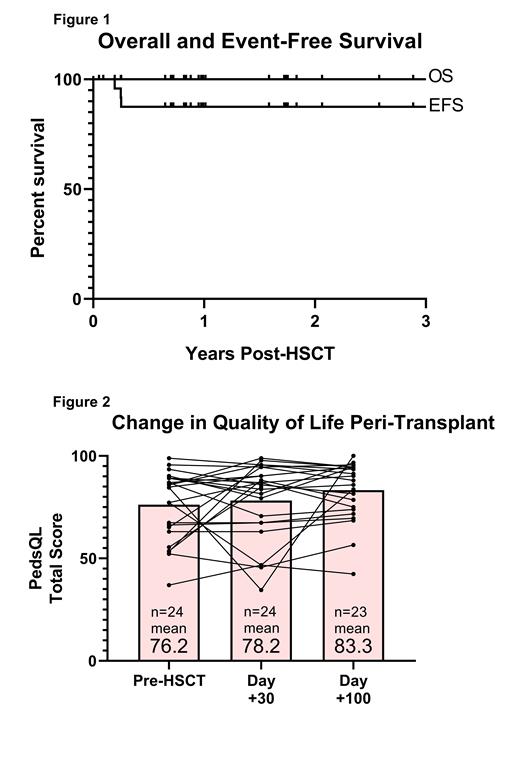
Results: All 30 of the patients planned for the primary endpoint of EFS have been enrolled. As of 7/20/2021, 24 patients are greater than 100 days post-HSCT with a median follow-up of 365 days (range 189-1105). The median age of this group at time of transplant was 13.5 years (range 2-22). All patients had neutrophil recovery (ANC >500/ul x 3 days) at a median of 21.5 days (range 0-29). Eleven patients (46%) did not require any platelet transfusions. Median transplant hospitalization was 16.5 days (range 14-43). Seven patients (29%) were again hospitalized (median 4 days, range 1-26). Three patients had secondary graft rejection at Day +71, +91, and +92 with no major complications and autologous recovery resulting in an EFS of 87.5% (Figure 1). Three additional patients have stable low donor chimerism (myeloid chimerism 11%, 38%, and 47%). The remaining 18 patients (75%) are disease-free and have robust donor engraftment (72-100% myeloid chimerism). Nine of these patients are >1 year post-HSCT and off sirolimus. Overall survival is 100% with no acute or chronic GVHD. Figure 2 shows total PedsQL scores during the peri-transplant time period. The median change in day +30 scores compared to baseline was 0 (IQR -7, +10), p=0.66. The median change in day +100 scores compared to baseline was +5 (IQR 0, +10), p=0.02.
Conclusions: Outcomes of pediatric nonmyeloablative HLA-identical sibling HSCT for SCD appear similar to the larger adult experience with a GVHD-free, rejection-free survival of >80%. Importantly, patients have no worsening of their quality of life in the first month post-HSCT and already an improvement at day +100, suggesting that the short-term toxicities experienced by patients transplanted with this regimen are minimal. The majority of patients achieve disease resolution with no GVHD, but graft failure incidence appears higher than with myeloablative HSCT. Given the low intensity of this approach, a reasonable strategy may be to reserve myeloablation for a second transplant in the minority of patients who experience graft failure.
Rangarajan: Medexus (Treosulfan): Consultancy, Honoraria. Guilcher: BlueBirdBio: Research Funding; Project Sickle Cure Study: Other: Principal Investigator, Research Funding.
alemtuzumab and sirolimus were used off-label as part of the studied transplant regimen